You are viewing the article 8 how many neutrons does sodium have Ultimate Guide at Tnhelearning.edu.vn you can quickly access the necessary information in the table of contents of the article below.
You are learning about how many neutrons does sodium have. Here are the best content by the team chuyendoi.top synthesize and compile, see more in the section How.
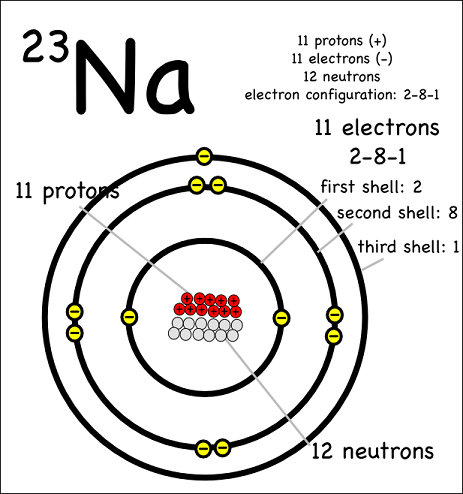
National 5 Chemistry Revision [1]
Atoms are made from protons, neutrons and electrons. In this study guide, you can revise how the periodic table arranges elements according to their atomic size and other properties, atomic theory and atomic numbers, you’ll also learn about isotopes and the properties of the main groups of elements.
This is written at the bottom left hand side of the symbol.. Since atoms are neutral, we know then that sodium atoms must also have 11 negative electrons to cancel the charge from 11 positive protons.
These energy levels can only hold a certain number of electrons.. The first energy level (the one nearest the nucleus) can hold a maximum of two electrons with the others being able to hold up to a maximum of 8 electrons (only true for the first 20 elements).
Sodium atoms have 11 electrons but 12 neutrons. Will it have any charge? [2]
Which of the following statement is correct about the atom of an element ?. (a) an atom can have only protons and neutrons but no electrons.
(c) an atom can have only electron and proton but no neutron. (d) an atom must always have a proton, neutron and electron
(b) They have same number of electrons but different number of neutrons. (c) They have same number of neutrons but different number of electrons
How many protons, neutrons and electrons does sodium have? [3]
How many protons, neutrons and electrons does sodium have?. Sodium is a classified alkali metal element and its symbol is Na
The atomic number of an element is equal to the number of protons and electrons in that element.. Therefore, a sodium atom has eleven protons and eleven electrons
The difference between the mass number of the sodium atom and the number of protons is twelve. The number of neutrons depends on the isotope of the element
Sodium – Protons – Neutrons – Electrons – Electron Configuration [4]
Sodium is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table, because it has a single electron in its outer shell that it readily donates, creating a positively charged atom—the Na+ cation.
Employed only in rather specialized applications, only about 100,000 tonnes of metallic sodium are produced annually. Sodium is now produced commercially through the electrolysis of molten sodium chloride.
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Multiple choice questions [5]
Crowe & Bradshaw: Chemistry for the Biosciences 3e Chapter 2: Multiple choice questions Instructions Answer the following questions and then press ‘Submit’ to get your score. Question 1 An atom of sodium has an atomic number of 11 and a mass number of 23
b) An atom of sodium has 11 protons, 12 electrons, and 11 neutrons. c) An atom of sodium has 11 protons, 11 electrons, and 12 neutrons
Question 2 An atom of phosphorus has an atomic number of 15 and a mass number of 31. How many neutrons does it contain? a) 15 b) 16 c) 31 Question 3 What is the chemical symbol for magnesium? a) Ma b) Mn c) M d) Mg Question 4 Complete the following sentence: ‘Different isotopes of the same element have…’ a) …the same number of protons, but differing numbers of neutrons and electrons b) …the same number of neutrons, but differing numbers of protons and electrons c) …the same number of protons and neutrons, but differing numbers of electrons d) …the same number of protons and electrons, but differing numbers of neutrons Question 5 Shell three contains how many sub-shells? a) 1 b) 2 c) 3 d) 4 Question 6 The 3d subshell can hold a maximum of how many electrons? a) 1 b) 2 c) 6 d) 8 e) 10 Question 7 Calcium has an atomic number of 20
How many neutrons does sodium have? -Turito [6]
atomic mass = number of protons + number of neutrons.. Atomic number = number of protons, atomic mass = number of protons + number of neutrons.
How many electrons can fit in the second or third orbit?. How many electrons can fit in the second or third orbit?
What is the maximum amount of electrons that can fit in the first orbit of a Bohr-Diagram?. Which of the following best describes the Bohr model of an atom?
How Many Protons Does Sodium Have [7]
In chemistry, to understand the chemical behaviour, reactivity, physical nature, and symmetry of an element, we study its subatomic particles, their numbers, their strength, and the overall dominant charge. If you don’t know then subatomic particles are the fundamental constituents of an atom.
The number of protons present in an atom is known as its atomic number whereas the total number of protons and neutrons an atom contains is called its atomic mass.. Chemists arrange all the elements, discovered up till now, into a periodic table based on their atomic numbers so if you know the atomic number of an element, you can predict a lot about its chemical nature by predicting its position in the table.
Physically, it’s very delicate that you can even cut it with a knife while chemically, it reacts vigorously with water. Why does this metal possess such a contrary nature? To know this, we need to find out the number of protons in sodium and number of neutrons in sodium present in atom’s nucleus.
2.4: Neutrons: Isotopes and Mass Number Calculations [8]
2.4: Neutrons: Isotopes and Mass Number Calculations. The final subatomic particle was not discovered until 1932
Therefore, any remaining subatomic particles must be uncharged, so as to not upset this established charge balance. Indeed, neutrons, which were named as a result of their neutral charge, do not possess any electrical properties
Neutrons are also located in the nucleus of an atom, and the mass of a neutron was found to be just slightly greater than the mass of a proton.. Each subatomic particle exists to serve a specific purpose
How to find the Number of Protons, Electrons, Neutrons for Sodium (Na)
How to find the Number of Protons, Electrons, Neutrons for Sodium (Na)
How to find the Number of Protons, Electrons, Neutrons for Sodium (Na)
Reference source
- https://www.bbc.co.uk/bitesize/guides/zw2gpbk/revision/4#:~:text=Since%20sodium%20has%2011%20protons,23%20%E2%80%93%2011%20%3D%2012%20neutrons.
- https://byjus.com/question-answer/sodium-atoms-have-11-electrons-but-12-neutrons-will-it-have-any-charge/#:~:text=Sodium%20atoms%20have%2011%20electrons%20but%2012%20neutrons.
- https://valenceelectrons.com/sodium-protons-neutrons-electrons/
- https://material-properties.org/sodium-protons-neutrons-electrons-electron-configuration/
- http://global.oup.com/uk/orc/biosciences/chembio/crowe3e/student/mcqs/ch02/
- https://www.turito.com/ask-a-doubt/Chemistry-how-many-neutrons-does-sodium-have-22-12-23-11-q5adc9323
- https://equationbalancer.com/blog/how-many-protons-does-sodium-have
- https://chem.libretexts.org/Courses/Heartland_Community_College/CHEM_120%3A_Fundamentals_of_Chemistry/02%3A_Atoms_and_Elements/2.04%3A_Neutrons%3A__Elemental_Isotopes_and_Mass_Number_Calculations
20 how to find ancient debris Ultimate Guide
Thank you for reading this post 8 how many neutrons does sodium have Ultimate Guide at Tnhelearning.edu.vn You can comment, see more related articles below and hope to help you with interesting information.
Related Search:

