You are viewing the article 4.10: Polar Molecules at Tnhelearning.edu.vn you can quickly access the necessary information in the table of contents of the article below.
4.10: Polar Molecules
- Page ID
- 86624
Learning Objectives
- Recognize bond characteristics of covalent compounds: bond length and bond polarity.
- Use electronegativity values to predict bond polarity.
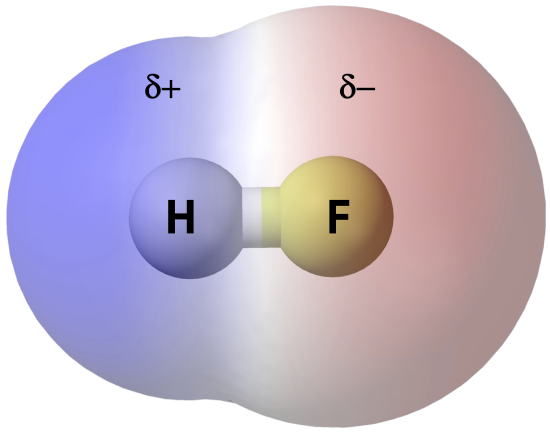
If there is only one bond in the molecule, the bond polarity determines the molecular polarity. Any diatomic molecule in which the two atoms are the same element must be a nonpolar molecule. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule where one end of the molecule is slightly positive, while the other end is slightly negative. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. Hence, a molecule with two poles has a dipole moment.
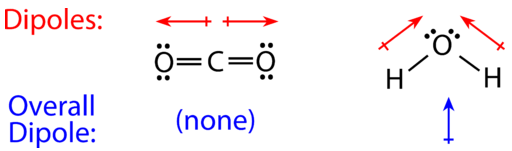
For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. The figure below shows a comparison between carbon dioxide and water.
Carbon dioxide (left( ce{CO_2} right)) is a linear molecule with carbon in the center and two oxygens at the terminal ends. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the (ce{C}) atom to each (ce{O}) atom. However, since the dipoles are of equal strength and pointing in opposite directions, they cancel out and the overall molecular polarity of (ce{CO_2}) is zero (no net dipole), therefore (ce{CO_2}) is a nonpolar molecule.
Water has a bent molecular structure because it has four electron groups, two bonded groups and two lone electron groups on the central oxygen atom. The individual O–H bond dipoles point from the slightly positive (ce{H}) atoms toward the more electronegative (ce{O}) atom. Because of the bent shape, the dipoles, which are equal in strength, both point towards the oxygen atom and will not cancel each other out, therefore, the water molecule is polar. In the figure below, you can see that the oxygen end of the molecule is slightly negative and the hydrogen side is slightly positive, there is a separation of charge throughout the whole molecule, a net dipole (shown in blue) that points upward.
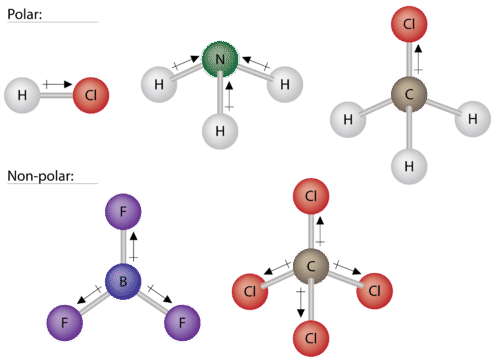
Some other molecules are shown in the figure below. Notice that a tetrahedral molecule such as (ce{CH_4}) is nonpolar. However, if one of the peripheral (ce{H}) atoms is replaced with another atom that has a different electronegativity, the molecule becomes polar. A trigonal planar molecule (left( ce{BF_3} right)) may be nonpolar if all three peripheral atoms are the same, but a trigonal pyramidal molecule (left( ce{NH_3} right)) is polar.
To summarize, to be polar, a molecule must:
- Contain at least one polar covalent bond.
- Have a molecular structure such that the sum of the vectors of each bond dipole moment do not cancel.
- Draw the Lewis structure.
- Figure out the geometry (using VSEPR theory).
- Visualize or draw the geometry.
- Find the net dipole moment (you don’t have to actually do calculations if you can visualize it).
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
Properties of Polar Molecules
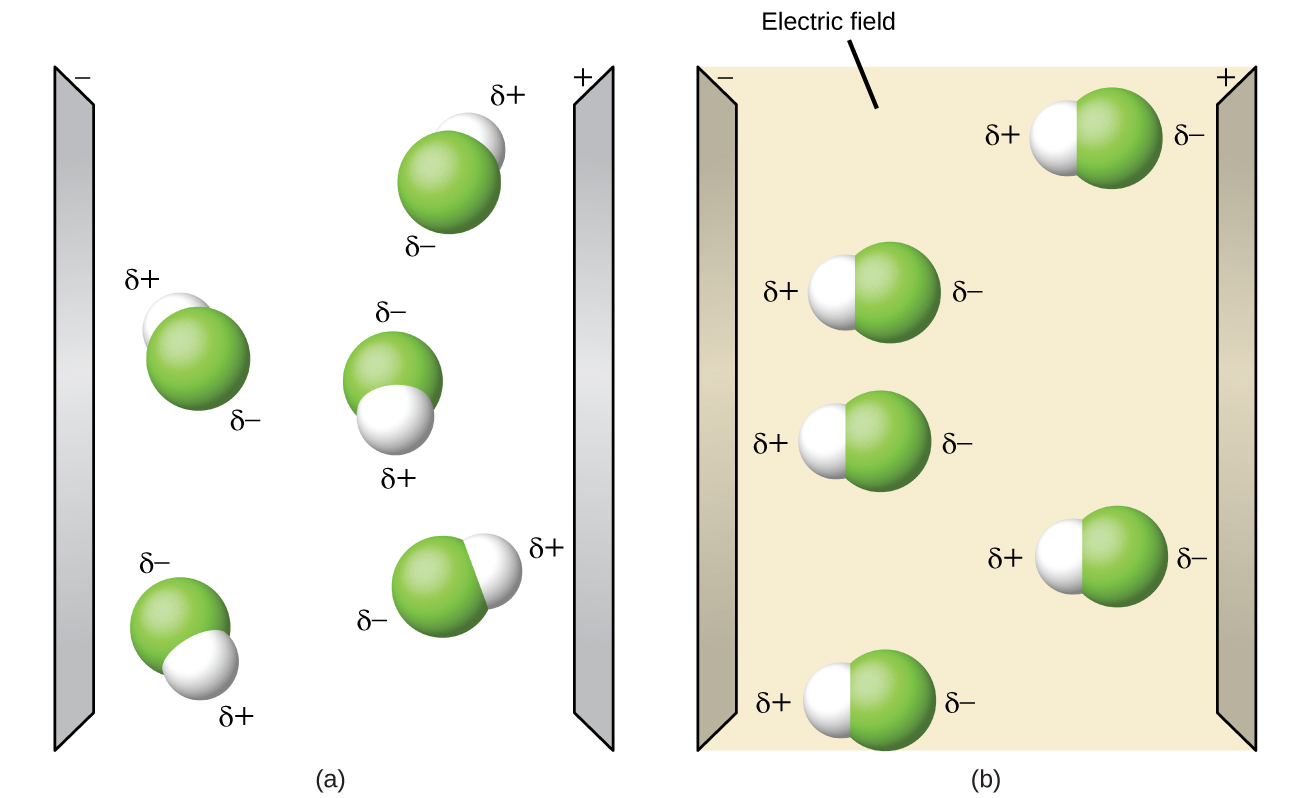
Polar molecules tend to align when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end toward the positive plate (Figure (PageIndex{4})). We can use an electrically charged object to attract polar molecules, but nonpolar molecules are not attracted. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances.
While molecules can be described as “polar covalent” or “ionic”, it must be noted that this is often a relative term, with one molecule simply being more polar or less polar than another. However, the following properties are typical of such molecules. Polar molecules tend to:
- have higher melting points than nonpolar molecules
- have higher boiling points than nonpolar molecules
- be more soluble in water (dissolve better) than nonpolar molecules
- have lower vapor pressures than nonpolar molecules
The following simulation is very useful in exploring more about bond and molecular polarity. Explore on your own or use the exercise and examples below.
Open the molecule polarity simulation (above) and select the “Three Atoms” tab at the top. This should display a molecule ABC with three electronegativity adjustors. You can display or hide the bond moments, molecular dipoles, and partial charges at the right. Turning on the Electric Field will show whether the molecule moves when exposed to a field.
Use the electronegativity controls to determine how the molecular dipole will look for the starting bent molecule if:
- A and C are very electronegative and B is in the middle of the range.
- A is very electronegative, and B and C are not.
Solution
- Molecular dipole moment points immediately between A and C.
- Molecular dipole moment points along the A–B bond, toward A.
Determine the partial charges that will give the largest possible bond dipoles.
- Answer
-
The largest bond moments will occur with the largest partial charges. The two solutions above represent how unevenly the electrons are shared in the bond. The bond moments will be maximized when the electronegativity difference is greatest. The controls for A and C should be set to one extreme, and B should be set to the opposite extreme. Although the magnitude of the bond moment will not change based on whether B is the most electronegative or the least, the direction of the bond moment will.
Thank you for reading this post 4.10: Polar Molecules at Tnhelearning.edu.vn You can comment, see more related articles below and hope to help you with interesting information.
Related Search:

